Actinide coordination chemistry
Actinide Coordination Complexes for the (Electrocatalytic) Activation and Transformation of Small Molecules
In our efforts to activate small molecules of industrial and biological importance, we have turned our attention to coordinatively unsaturated, reactive uranium coordination complexes. For example, the chelating triazacyclononane (tacn), single N, and arene-anchored tris(aryloxide) ligands, (ArO)3tacn3–, (ArO)3N3–, (ArO)3mes3–, as well as the tetra(aryloxide) ligand (ArO)4cyclen4– and the monodentate Ad,Ad,MeArO– have provided access to reactive coordination compounds of uranium in oxidation states II, III, IV, V, and VI with tailorable steric and electronic profiles. These complexes display a pronounced selectivity and reactivity in reactions with carbon dioxide, related small heteroallene molecules (COS and CS2), NxO and H2O.
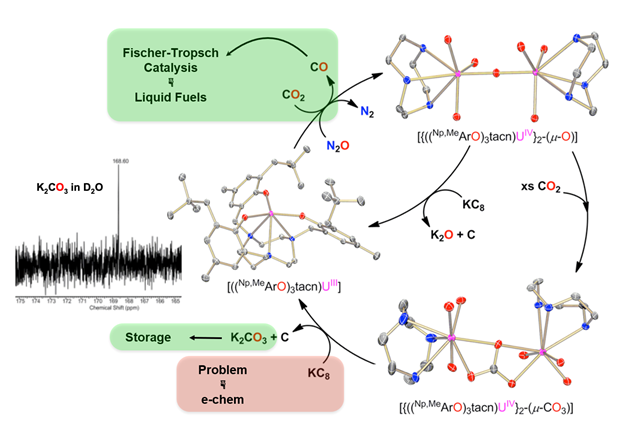
The stoichiometric and catalytic “disproportionation” of CO2 to CO and CO32– via reductive cleavage of CO2 was accomplished. In analogy, we were able to isolate an entire series of chalcogenide mixed-carbonate complexes by reacting bridged chalcogenide complexes U‒E‒U (E = S, Se, Te) with CO2, CS2, and COS. The corresponding terminal chalcogenide complexes U≡E (E = O, S, Se, Te) were obtained more recently by reaction with H2E and subsequent reduction.
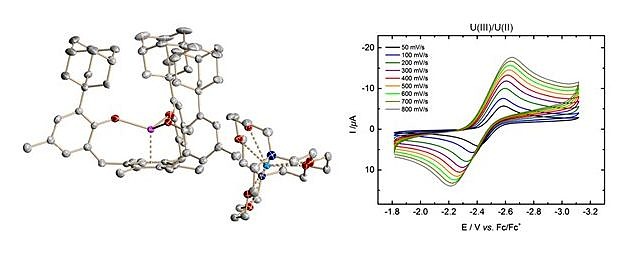
With respect to fundamental understanding of actinide chemistry, the molecular and electronic structure of a rare oxidation state in U coordination chemistry, namely U(II), with a 5f 4 quintet ground state was stabilized by a mesitylene anchored tris(aryloxide) ligand. In K(crypt)[((Ad,MeArO)3mes)U], the U(II) center is supported by d backbonding, a general motive of the (ArO)3mes3– ligand.
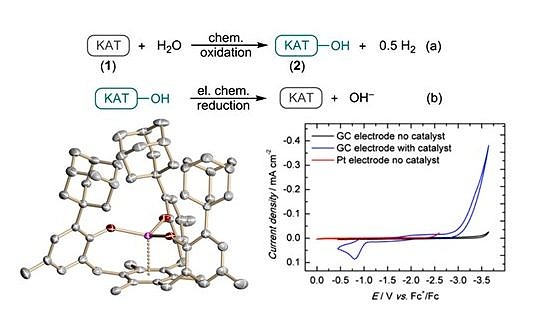
In analogy to the CO2 reduction, a rare U(IV) hydroxo complex, [((Ad,MeArO)3mes)U(OH)], with a terminal OH functionality was synthesized by selective reduction of H2O with the U(III) complex [((Ad,MeArO)3mes)U]. Mechanistic studies were performed to understand this fundamental reaction, establishing the basis of catalytic H2O reduction for H2 evolution. Comprehensive electrochemical studies revealed that the U(III) complex can be regenerated in a catalytic cycle combining chemical and electrochemical reaction steps to produce H2 from water.
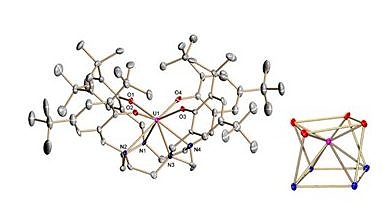
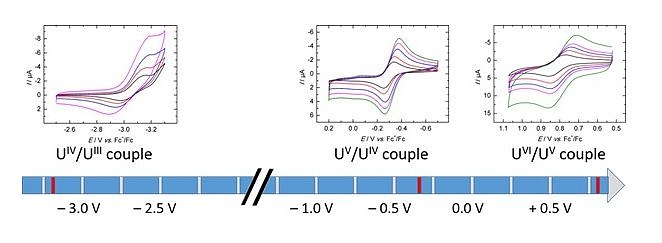
Complexation of the newly synthesized ligand (ArO)4cyclen4– provides remarkably rare water and air stable uranium(IV) and (V) complexes, with redox events ranging from tri- to hexavalent species and covering an electrochemical window of ~4V.
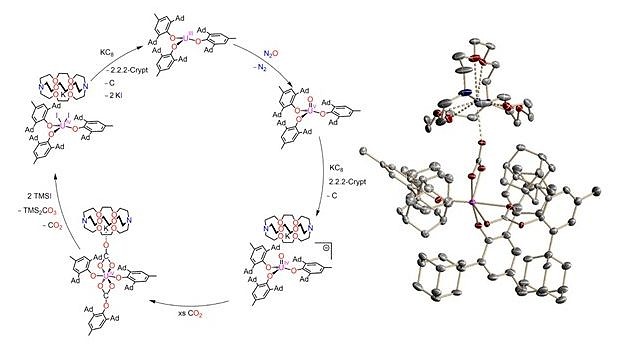
Reaction of the trivalent uranium complex [((Ad,MeArO)3N)U] with [Na(OCP)(dioxane)2.5] or [Na(OCAs)(dioxane)3] yielded the first example of a coordinated η1-cyaarside ligand (CAs–) and the unprecedented binding motif U–CP. The sterically demanding monodentate Ad,Ad,MeArO– ligand gives access to activation of N2O or NO and subsequent conversion of CO2 into the solid carbonate TMS2CO3, in a closed synthetic cycle
For details on our actinide chemistry program, please check out the following selection of references:
- H2O Activation and Electrocatalysis
D.P. Halter, F.W. Heinemann, J. Bachmann and K. Meyer
Uranium-Mediated Electrocatalytic H2 Production from Water
Nature 2016, 530, 317–321.
D.P. Halter, F.W. Heinemann, L. Maron and K. Meyer
The Role of Uranium-Arene Bonding in H2O Reduction Catalysis
Nature Chemistry 2018, 10, 259–267.
D.P. Halter, C.T. Palumbo, J.W. Ziller, M. Gambicki, A.L. Rheingold, W.J. Evans and K. Meyer
Electrocatalytic H2O Reduction with f-Elements: Mechanistic In-sight and Overpotential Tuning in a Series of Lanthanide Complexes
J. Am. Chem. Soc. 2018, 140, 2587–2594.
- CO2 Activation and Transformation
Castro-Rodriguez, H. Nakai, L. N. Zakharov, A.L. Rheingold and K. Meyer
A Linear, O-Coordinated eta1-CO2 Bound to Uranium
Science 2004, 305, 1757–1759.
S.C. Bart, C. Anthon, F.W. Heinemann, E. Bill, N.M. Edelstein and K. Meyer
Carbon Dioxide Activation with Sterically Pressured Mid- and High-Valent Uranium Complexes
J. Am. Chem. Soc. 2008, 130, 12536–12546.
A.-C. Schmidt, A.V. Nizovtsev, A. Scheurer, F.W. Heinemann and K. Meyer
Uranium-Mediated Reductive Conversion of CO2 to CO and Carbonate in a Single-Vessel Closed Synthetic Cycle
Chem. Comm. 2012, 48, 8634–8636.
O.P. Lam, S.M. Franke, F.W. Heinemann and K. Meyer
Reactivity of U-E-U (E = S, Se) Towards CO2, CS2 and COS: New Mixed-Carbonate Complexes of the Types U-CO2E-U (E = S, Se), U-CS2E-U (E = O, Se) and U-COSSe-U
J. Am. Chem. Soc. 2012, 134, 16877–16881.
Waldschmidt, C.J. Hörger, J. Riedhammer, F.W. Heinemann, C.T. Hauser and K. Meyer
CO2 Activation with Formation of Uranium Carbonate Complexes in a Closed Synthetic Cycle
Organometallics 2020, 39, 1602–1611.
- New and Unusual Oxidation States
H.S. La Pierre, H. Kameo, D.P. Halter, F.W. Heinemann and K. Meyer
Coordination and Redox Isomerization in the Reduction of a Uranium(III) Monoarene Complex
Angew. Chem. Int. Ed. 2014, 53, 7154–7157.
H.S. La Pierre, A. Scheurer, F.W. Heinemann, W. Hieringer and K. Meyer
Synthesis and Characterization of a Uranium(II) Monoarene Complex Supported by delta-Backbonding
Angew. Chem. Int. Ed. 2014, 53, 7158–7162.
- Small Molecule Activation Chemistry and Unusual Reactivity
A.-C. Schmidt, F.W. Heinemann, W.W. Lukens Jr. and K. Meyer
Molecular and Electronic Structure of Dinuclear Uranium di-µ-Oxo Complexes with Diamond Core Structural Motifs
J. Am. Chem. Soc. 2014, 136, 11980–11993.
S.M. Franke, F.W. Heinemann and K. Meyer
Reactivity of Uranium(IV) Bridged Chalcogenido Complexes UIV-E-UIV (E = S, Se) with Elemental Sulfur and Selenium: Synthesis of Polychalcogenido-Bridged Uranium Complexes
Chem. Sci. 2014, 5, 942–950.
S.M. Franke, M.W. Rosenzweig, F.W. Heinemann and K. Meyer
Reactivity of Uranium(III) with H2E (E = S, Se, Te): Synthesis of a Series of Mononuclear and Dinuclear Uranium(IV) Hydrochalcogenido Complexes”
Chem. Sci. 2015, 6, 275–282.
C.J. Hörger, F.W. Heinemann, E. Louyriac, M. Rigo, L. Maron, H. Grützmacher, M. Driess and K. Meyer
Cyaarside (CAs–) and 1,3-Diarsaallendiide (AsCAs2–) Ligands Coordinated to Uranium and Generated via Activation of the Arsaethynolate Ligand (OCAs–)
Angew. Chem. Int. Ed. 2019, 58, 1679–1683.
- Development of Custom-Tailored Ligands and Synthesis
J. Hümmer, F.W. Heinemann and K. Meyer
Uranium Tetrakis-Aryloxide Derivatives Supported by Tetraazacyclododecane: Synthesis of Air-Stable, Coordinatively-Unsaturated U(IV) and U(V) Complexes
Inorg. Chem. 2017, 56, 3201–3206.