Organometallic chemistry
Tripodal N-Heterocyclic Carbene Ligands and Their Reactive Transition Metal Complexes for Small Molecule Activation
 Transition metal coordination compounds in high oxidation states are active intermediates in many biocatalytic cycles. In this context, the study of high valent transition metal complexes featuring terminal, multiple bonded oxido (O2–) or nitrido (N3–) ligands is of significant and current interest due to their proposed intermediary in biological systems and industrial processes. Examples include O- and N-atom transfer chemistry, epoxidation and aziridination catalysis, water-splitting catalysis with Co-oxo species and biological ammonia synthesis performed by iron nitrido species. Spectroscopic evidence for these intermediates is often based on studies of well-defined inorganic coordination complexes modelling the natural analogues. The synthesis and characterization of high-valent metal oxo and nitrido model compounds is therefore often key to delineating the nature and reactivity of these unusually reactive species and assigning their role e.g. in water splitting catalysis and ammonia synthesis. Insight into the molecular and electronic structure of complexes that stabilize the intermediate [Fe≡N] entity appears to be valuable in unveiling the key steps of both biological (nitrogenase) and industrial (Haber-Bosch process) NH3 synthesis.
Transition metal coordination compounds in high oxidation states are active intermediates in many biocatalytic cycles. In this context, the study of high valent transition metal complexes featuring terminal, multiple bonded oxido (O2–) or nitrido (N3–) ligands is of significant and current interest due to their proposed intermediary in biological systems and industrial processes. Examples include O- and N-atom transfer chemistry, epoxidation and aziridination catalysis, water-splitting catalysis with Co-oxo species and biological ammonia synthesis performed by iron nitrido species. Spectroscopic evidence for these intermediates is often based on studies of well-defined inorganic coordination complexes modelling the natural analogues. The synthesis and characterization of high-valent metal oxo and nitrido model compounds is therefore often key to delineating the nature and reactivity of these unusually reactive species and assigning their role e.g. in water splitting catalysis and ammonia synthesis. Insight into the molecular and electronic structure of complexes that stabilize the intermediate [Fe≡N] entity appears to be valuable in unveiling the key steps of both biological (nitrogenase) and industrial (Haber-Bosch process) NH3 synthesis.
The Meyer group’s approach to high-valent complexes is based on our signature tripodal NHC ligands, which provide a powerful platform for small molecule activation chemistry. This is due to the ligand field resulting from the trigonal carbene environment enabling the stabilization of the desired and highly unusual metal to ligand multiple bonds.
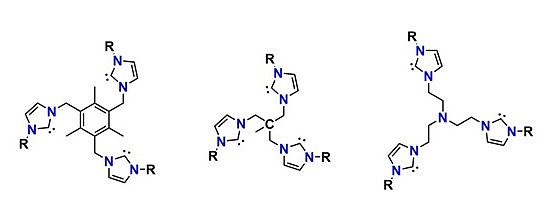
Chelating tris(carbene) ligands employed by the Meyer Group
We have discovered that employing tripodal N-heterocyclic carbene chelates developed by us, allows for the synthesis, isolation as well as the structural and spectroscopic characterization of discrete mid- and high valent imido, nitrido, and peroxo complexes.
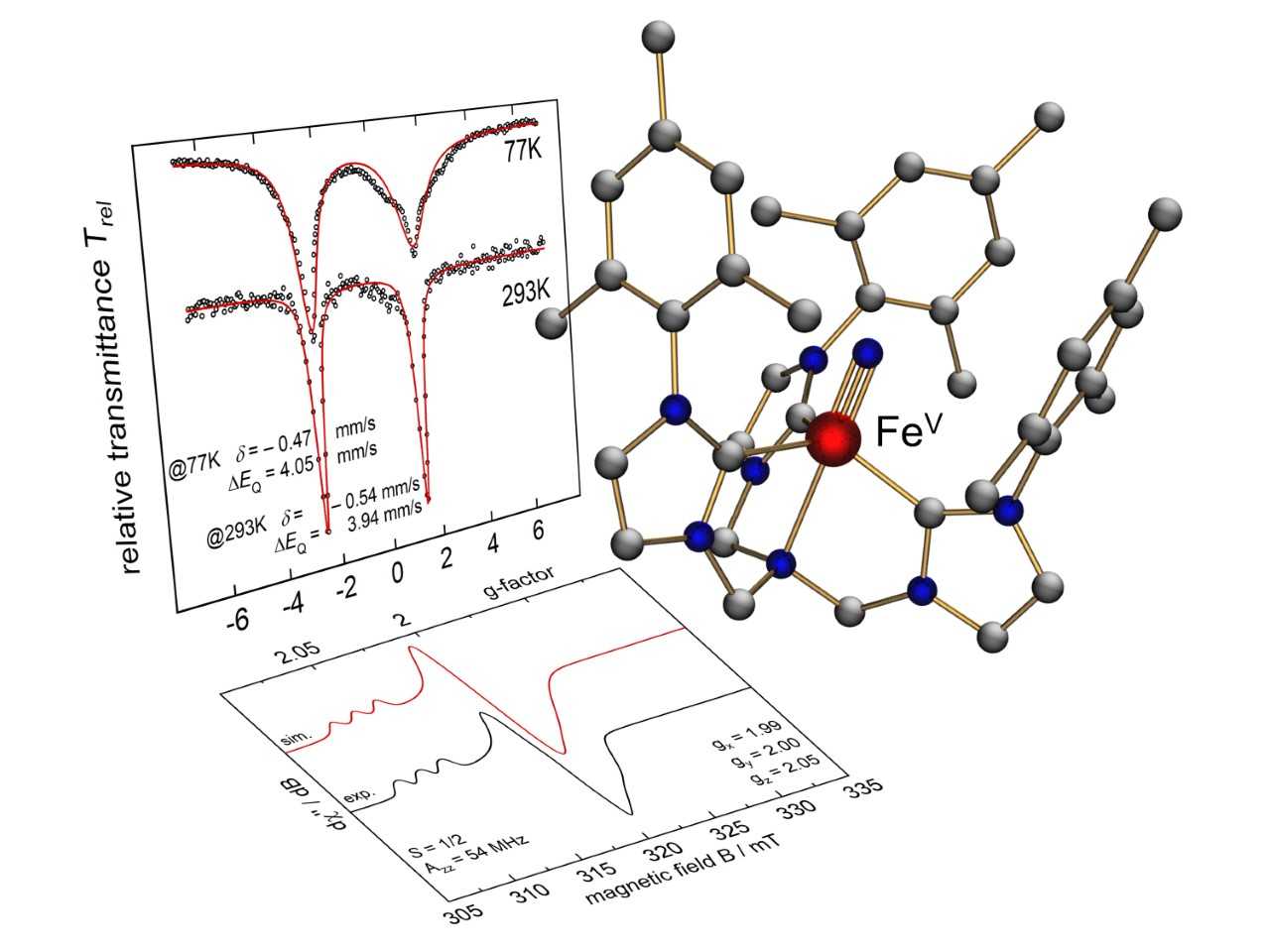
With these ligands in hand, we successfully prepared and fully characterized a series of high- and mid-valent manganese, iron, and cobalt nitrido complexes. The X-ray diffraction analyses of iron(IV) and iron(V) nitrido complexes were the first structural verification of the [Fe≡N] synthon that has been recognized as the reactive intermediate in molecular ammonia synthesis. Reactivity studies of the Fe(V) species include the reaction of an FeV≡N with water to yield ammonia in near quantitative yields.
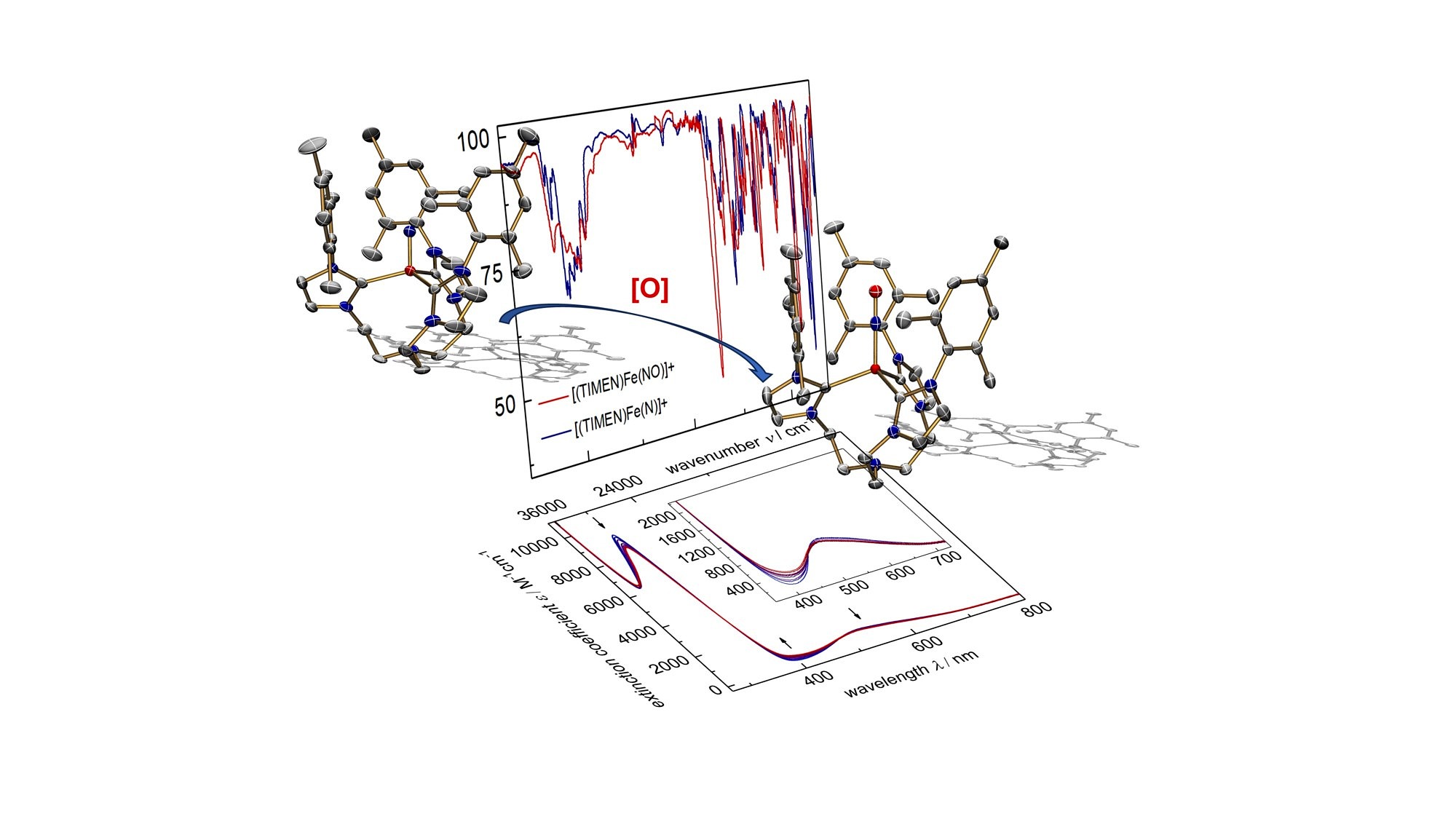
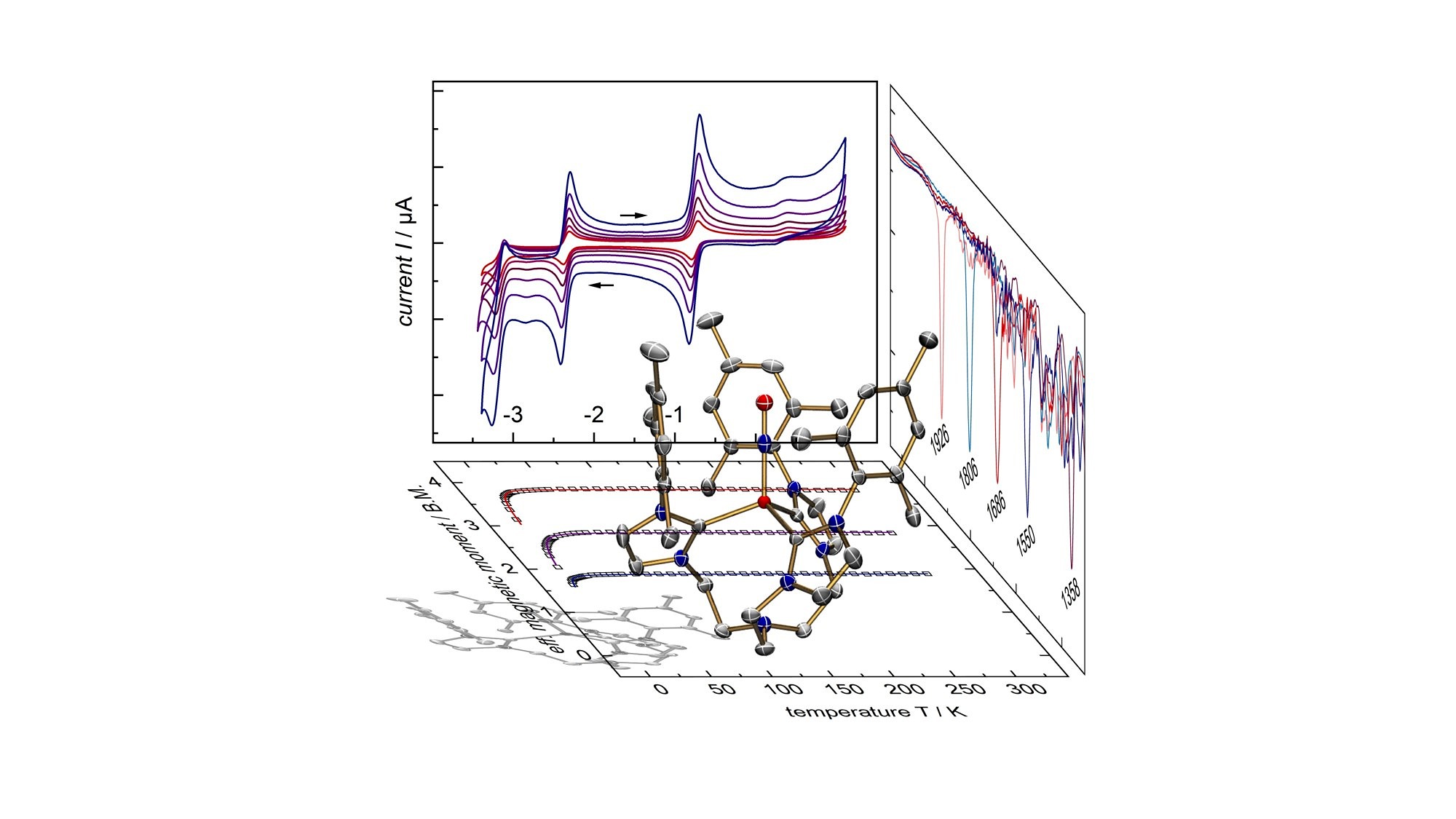
Oxgenation of an iron nitride species allowed for the synthesis of an iron nitrosyl species exhibiting an interesting electrochemical behavior. Iron-Nitrosyls have fascinated chemists for a long time due to the non-innocent nature of the NO ligand that can exist in up to five different oxidation and spin states. Coordination to an open-shell iron center leads to complex electronic structures. In our studies, we succeeded to characterize a series of {Fe-NO}6-9 complexes, including a reactive {Fe-NO}10 intermediate.
For details on our transition metal chemistry program, please check out the following selection of references:
C. Vogel, F.W. Heinemann, J. Sutter, C. Anthon and K. Meyer
An Iron Nitride Complex
Angew. Chem. Int. Ed. 2008, 47, 2681–2684.
J.J. Scepaniak, C.S. Vogel, M.M. Khusniyarov, F.W. Heinemann, K. Meyer and J.M. Smith
Synthesis, Structure, and Reactivity of an Iron(V) Nitride
Science 2011, 331, 1049–1052.
H. Kropp, A.E. King, M.M. Khusniyarov, F.W. Heinemann, K.M. Lancaster, S. DeBeer, E. Bill and K. Meyer
Manganese Nitride Complexes in Oxidation States III, IV, and V: Synthesis and Electronic Structure
J. Am. Chem. Soc. 2012, 134, 15538–15544.
E.M. Zolnhofer, M. Käß, M.M. Khusniyarov, F.W. Heinemann, L. Maron, M. van Gastel, E. Bill and K. Meyer
An Intermediate Cobalt(IV) Nitrido Complex and its N-Migratory Insertion Product
J. Am. Chem. Soc. 2014, 136, 15072–15078.
M. Keilwerth, J. Hohenberger, F.W. Heineman, J. Sutter, A. Scheurer, H. Fang, E. Bill, F. Neese, S. Ye and K. Meyer
A Series of Iron Nitrosyl Complexes {Fe–NO}6–9 and a Fleeting {Fe–NO}10 Intermediate en Route to a Metalacyclic Iron Nitrosoalkane
J. Am. Chem. Soc. 2019, 141, 17217–17235.
M. Keilwerth, L. Grunwald, W. Mao, F.W. Heineman, J. Sutter, E. Bill and K. Meyer
Ligand Tailoring Toward an Air-Stable Iron(V) Nitride Complex
J. Am. Chem. Soc. 2021, 143, 1458–1465.